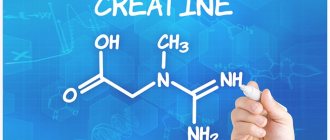
Structure of basic amino acids: 20 “magic” ones that make up protein. Structure. Classifications. Tables with formulas. Name and international abbreviations of proteinogenic amino acids. I am with you, Galina Baeva, 20 “magic” amino acids and beautiful tables with structural formulas of natural amino acids.
Natural amino acids are structural units (monomers) of proteins. Proteins contain only 20 kn. "magic" amino acids, which are also called proteinogenic. They all have a similar structure.
In addition to proteinogenic amino acids, the body also contains non-proteinogenic amino acids that perform various jobs, mainly intermediate compounds in the biochemical pipeline, such as ornithine, signaling molecules such as β-alanine or neurotransmitters such as GABA.
Features of the structure of natural amino acids
The structure of amino acids is closely related to their functions. Substances with similar chemical structures do similar work. Let’s try to figure it out so that we don’t get confused later in the drug annotations.
All amino acids are molded according to one pattern.
The head is an amine residue containing nitrogen N.
A carbon skeleton consisting of a chain of carbon atoms (in the simplest case, one carbon, to which an amine residue is attached “in front” and a carbon tail at the back)
Tail – carboxylic acid residue – COOH
Some other chemical group may be attached to the side of the carbon skeleton, which gives the substance special properties.
The carbon chain, together with the acid tail, attached to the amine head is called an aliphatic radical.
How to count the quantity
How many amino acids are in a protein is determined by the protein itself. In a complete protein (that’s what it’s called - complete) the entire aminocarbon composition is found. In a defective person, several AMKs are missing. In addition, proteins can be simple (they contain only amino acids) or complex (the amino acid “complex” is supplemented with other chemical compounds). But in all cases, it is aminocarboxylic acids that are the basis for the construction of the protein molecule, and without these substances the proper functioning of the body is impossible.
Nomenclature of amino acids
The carbon chain (skeleton) can consist of either 1 carbon atom or several. In the latter case, it matters which carbon atom, starting from the carboxyl group, the amine head is attached to. This can be either the 1st carbon atom, or the 2nd, 3rd and so on. Chemists agreed to designate carbon atoms not by numbers, but by letters of the Greek alphabet: α is the 1st carbon atom, starting with the carboxyl tail, β is the 2nd, γ is the 3rd, etc.
If an amino group is attached to a carbon in the α-position, such an amino acid is called an α-amino acid, respectively, if the amino group is attached in the β-position, then it is a β-amino acid, if in γ, then it is a γ-amino acid.
All 20 natural proteinogenic amino acids belong to the group of α-amino acids.
Of the β-amino acids, the most famous is β-alanine, and of the γ-amino acids, the most famous is γ-aminobutyric acid (GABA). Their structural formulas are given below.
Classification of amino acids
There are several classifications of amino acids:
- Depending on the structure of the aliphatic radical, amino acids are divided into the following groups:
- Just amino acids with an aliphatic radical, i.e. those in which the carbon chain does not contain additional tricks. They are called M ono A mino M ono K arbonic acids: glycine and alanine
- Amino acids with a branched side chain, in which the carbon skeleton forms lateral forks: valine, leucine, isoleucine . Isoleucine is indistinguishable from leucine in chemical composition, but its carbon skeleton is curved differently, i.e. it is a stereoisomer. Sometimes it is isolated into a separate amino acid, and sometimes not. Amino acids with a branched side chain also belong to the group of M ono A mino M ono K arboxylic amino acids.
- Amino acids that have different groups in the aliphatic radical:
Alcohol - OH. They are called O xy M ono A mino M ono K arbonic acids: serine and threonine
Carboxyl , i.e. second acid tail. These are M on A mino D and K arboxylic amino acids: aspartic acid (aspartate) and glutamic acid (glutamate). They are also called acidic amino acids, a kind of “butter oil”.
Amide . The carboxyl tail has grown a second amine head: asparagine and glutamine . It seems clear that these are derivatives of aspartate and glutamate, respectively. They are called Amides M ono A mino D and K arboxylic amino acids
Amine . A second amine head has been added to the carbon skeleton: lysine
Guanidine : additional amine inserts - arginine
Lysine and Arginine are also classified as group D and A mino M ono K arboxylic amino acids, because they each have a second amine group. Since these amino acids in a neutral environment (water, pH = 7), exhibit alkaline (basic) properties, increasing the pH value (pH becomes › 7), they are classified as essential amino acids
Sulfur-containing amino acids. Have a sulfur atom S : cysteine, methionine
Amino acids containing an aromatic radical - a carbon ring or Aromatic amino acids phenylalanine, tyrosine, tryptophan
Amino acids with a heterocyclic radical are a ring with a nitrogen atom instead of a carbon, so it is “hetero” - “diverse”: tryptophan and histidine.
It is easy to notice that tryptophan is included in the group of both aromatic amino acids and in the group of amino acids with a heterocyclic radical, and all because it has both a heterocyclic radical and an aromatic one.
Imino acids - the carbon skeleton is not extended into a chain, but is closed in a ring, from which the amine head and the acid tail protrude nearby: proline and hydroxyproline
2. Classification based on the polarity of the aliphatic radical.
- Nonpolar (hydrophobic) amino acids . They have non-polar bonds between CC and CH atoms. These are glycine, alanine, valine, leucine, isoleucine, proline, tryptophan - 8 amino acids
- Polar uncharged (hydrophilic) amino acids . They have polar bonds between the atoms C-O, CN, OH, SH. These are serine, asparagine, glutamine, threonine, methionine - 5 amino acids
- Polar negatively charged amino acids . Their radical contains groups that are negatively charged in an aqueous environment (pH = 7), i.e. they act as a negatively charged ion (anion). These are aspartic and glutamic acids, tyrosine, cysteine - 4 amino acids
- Polar positively charged amino acids . Their radical contains groups that are positively charged in an aqueous environment (pH = 7), i.e. they act as a positively charged ion (cation). These are lysine, arginine, histidine - 3 amino acids.
The more amino acids that have polarity in a protein, the higher the protein’s ability to undergo chemical reactions, i.e. its reactogenicity. The reactogenicity of a protein is directly related to its functions. Connective tissue proteins, such as keratin, which is part of hair and nails, have few polar amino acids. On the contrary, enzymes are proteins that catalyze biochemical reactions and have an amino acid composition with many polar groups.
3. Classification in relation to pH value
- Amino acids with neutral properties with a pH of 5.97 - 6.02 . These are glycine, alanine, serine, valine, leucine, isoleucine, threonine, cystine, methionine - 9 amino acids. They have one amine head and one carboxyl tail
- Amino acids with slightly acidic properties pH 3.0 - 5.7 . These are aspartic and glutamic acids. They have one amine head but two carboxyl tails, which is why they are called "acids".
- Amino acids with alkaline properties with a pH of 9.7 - 10.7 . They have two amine heads and one carboxyl tail. These are lysine, arginine, histidine.
4. Classification according to the ability to synthesize in humans and animals.
- Essential amino acids: glycine, serine, alanine, aspartic acid, asparagine, glutamic acid, glutamine, proline
- Conditionally essential amino acids: arginine, histidine, tyrosine, cysteine
- Essential amino acids: valine, leucine, isoleucine, threonine, lysine, tryptophan, phenylalanine, methionine
They are described in more detail here: Replaceable and irreplaceable amino acids: where to get them.
5. Classification of amino acids according to biosynthetic pathways.
In living organisms, amino acids can be produced (synthesized) from other compounds. The biosynthetic pathway is a sequence of chemical reactions that are determined by the hereditary (genetic) matrix. It is written in the genetic code and is due to the presence of enzymes that trigger these reactions. Biosynthesis is not chaotic, and the number of starting and intermediate compounds is limited. Thus, out of the entire variety of natural amino acids, only 20 are used for protein synthesis. Accordingly, the initial and intermediate compounds in the biosynthesis pathways of individual amino acids form clusters or families where the compounds can be converted into each other.
- Aspartate family: aspartic acid (aspartate), asparagine, isoleucine , lysine , threonine, methionine
- Glutamate family: glutamic acid (glutamate), glutamine, proline, arginine
- Pyruvate family: alanine, valine , leucine
- Serine family: serine, glycine, cysteine
- Pentose family: histidine, tryptophan, phenylalanine , tyrosine
- Shikimate family: tryptophan, phenylalanine , tyrosine
It must be said that these metabolic pathways are implemented in biological systems, but not all of them are present in the human body. Thus, higher animals and humans are not able to synthesize the aromatic ring, so the shikimat path is not for us. Similar to other pathways for the synthesis of essential amino acids. For clarity, essential amino acids are shown bold .
6. Classification of amino acids by catabolic pathways
Catabolism is a process of breakdown and is the opposite of anabolism or the process of synthesis. In the body, catabolism is also determined by the genetic program and a set of enzymes. The end result of amino acid degradation is ammonia, water and carbon dioxide, and energy is released in the form of heat or bound in ATP molecules. Depending on the intermediate compounds that provide energy, amino acids are divided into the following groups:
- Glucogenic: producing metabolites (intermediates) from which glucose can be synthesized: glycine, alanine, serine, threonine, valine, aspartic acid, asparagine, glutamic acid, glutamine, proline, arginine, histidine, cystine, methionine
- Ketogenic: breaking down to acetoacetyl CoA and acetyl CoA, from which ketone bodies can be synthesized: lysine, leucine
- Intermediate: the breakdown of these amino acids produces metabolites of both types: isoleucine, tryptophan, phenylalanine, tyrosine
You can read more about glucogenic and ketogenic amino acids here: Glycogenic amino acids
Daily norm
A healthy body must receive all the necessary amino acids
For each amino acid there is a certain daily requirement:
- For Valin it is 2.5 grams.
- For Isoleucine – 2 grams.
- Leucine requires 4.5 grams.
- The daily norm of Lysine is 4 grams.
- Methionine requires about 2 grams per day.
- The appropriate dosage of Tyrosine is 4.4 grams.
- Threonine must be consumed per day 2.4 grams.
- Tryptophan is enough and about 1 gram.
- Phenylaniline is required in a dosage of 4.4 grams.
Right and left amino acids
Depending on the attachment of the amino group in relation to the carboxyl tail in the carbon chain, amino acids can be “right-handed” or “left-handed”, in other words, they are classified as D- or L-isomers. Such forms are called optically active; they do not differ in chemical composition, but in space they relate to each other, like the left and right hand.
In protein molecules, only L (left) isomers of amino acids are present, right (D) isomers can have special properties and act as mediators, i.e. signaling molecules, but more often they form ballast. There are practically no D-amino acids in regular foods. They are formed during chemical synthesis and can be found in artificial proteins used in sports nutrition or as dietary supplements. D-amino acids are difficult to break down by enzymes because they are not physiological. The liver and kidneys contain a special enzyme, D-amino acid oxidase, which is believed to convert non-physiological right-handed amino acids into physiological left-handed ones. Its quantity is small, because Foods typically contain very few D-amino acids.
During chemical synthesis, equal amounts of D- and L-isomers are formed, but only L-series amino acids participate in protein synthesis. This should be taken into account by people taking amino acid preparations: L-amino acids will be significantly more expensive due to the need to isolate them from the mixture, but the effect of their use will be significantly higher
Read on to learn what each amino acid does in the body. Believe me, they have something to do. Galina Baturo was with you. Share information on social networks, leave comments.
In what form are they produced?
Dietary supplements are produced both in the form of individual amino acids and in the form of complexes designed to solve specific problems.
Amino acid supplements come in 4 forms:
- In capsules
- In tablets
- Powder
- In liquid form
Amino acids are very easy to identify. The word Amino is written on the packaging.
The form of release of amino acid complexes has more to do with convenience than with the principle and speed of action.
The powder is quickly absorbed and allows the athlete to measure the dosage he needs. But such a product needs to be prepared and is inconvenient to take with you for a run or to the gym.
Capsules and tablets win in this regard - they are very convenient and easy to store. But there is a very small concentration of amino acids per capsule or tablet. Therefore, sometimes you have to take them by the handful.
The liquid form is absorbed even better and faster, but has a short shelf life, high price and special storage conditions.